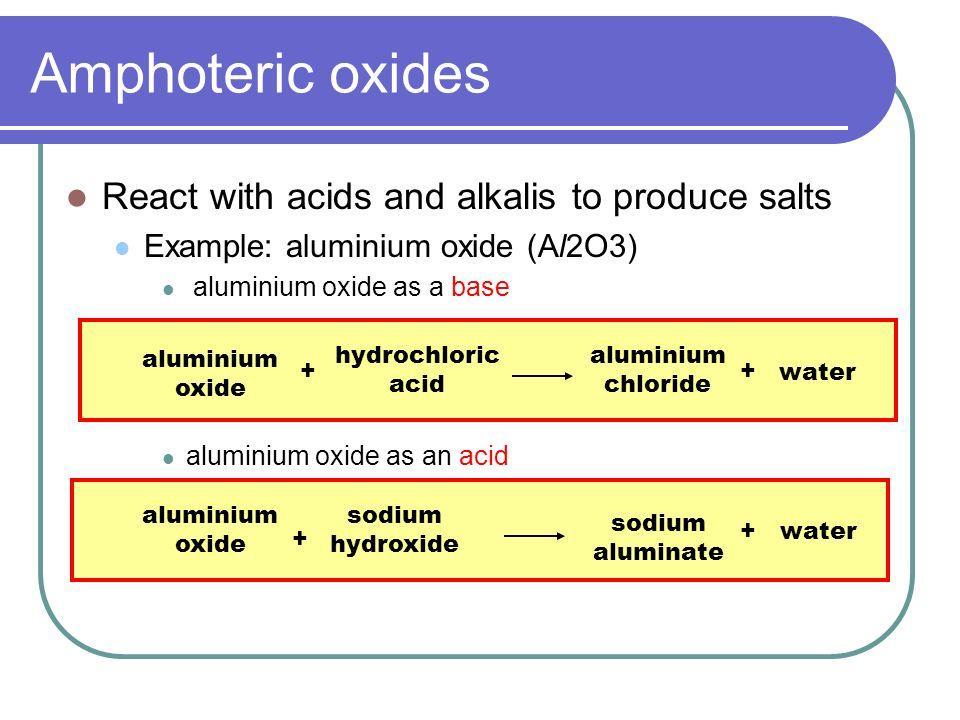
What do you call the metal oxide having both acidic and basic properties. Give two examples. Give - Brainly.in
Why are some amphoteric oxides, what's the root reason for it? Please don't reply that they react with both acid and base, what's the chemical reason for it? - Quora

Al2O3+HCl=AlCl3+H2O Balanced Equation||Aluminium oxide + Hydrochloric acid Balanced Equation - YouTube

Title: Lesson 4 Period 3 Oxides Learning Objectives: Understand and explain the trend in acid-base behaviour of the period 3 oxides Complete an experiment. - ppt download
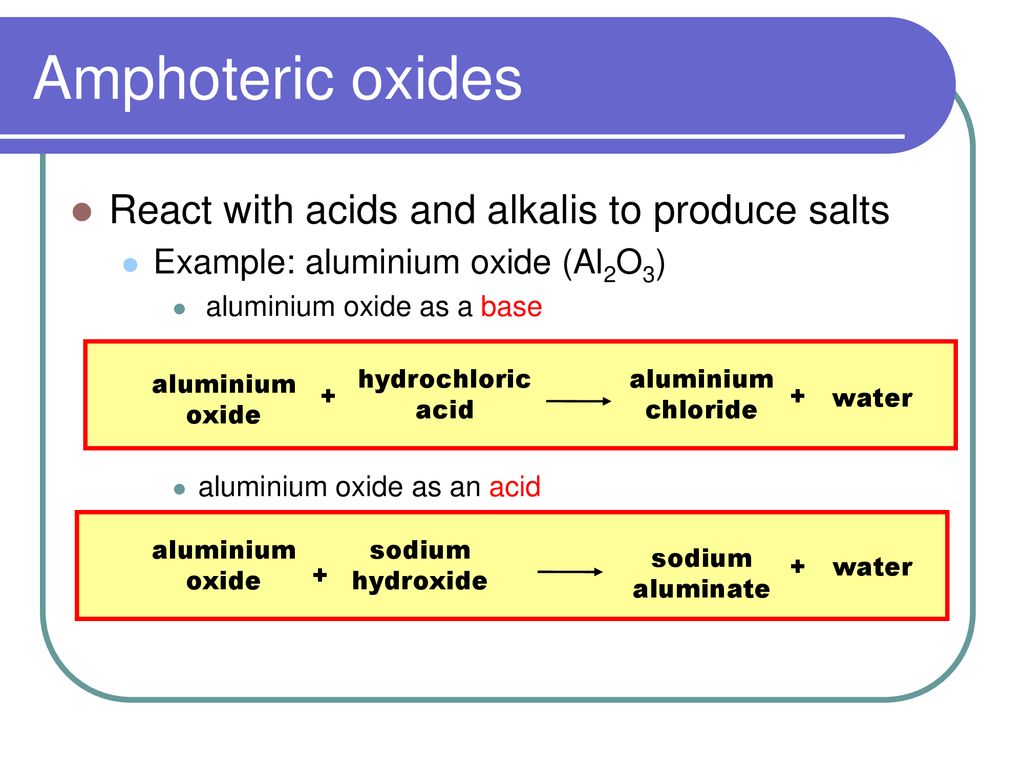
What is an oxide? An oxide is a Binary compound of oxygen and another element. M & O Oxides can be classified in two ways – Nature of Oxides Amount of. -

SOLVED: The balanced chemical equation for the reaction between aluminum oxide and sulfuric acid is: Al2O3 ( s ) + 3 H2SO4 ( aq ) Al2(SO4)3 ( aq ) + 3 H2O (
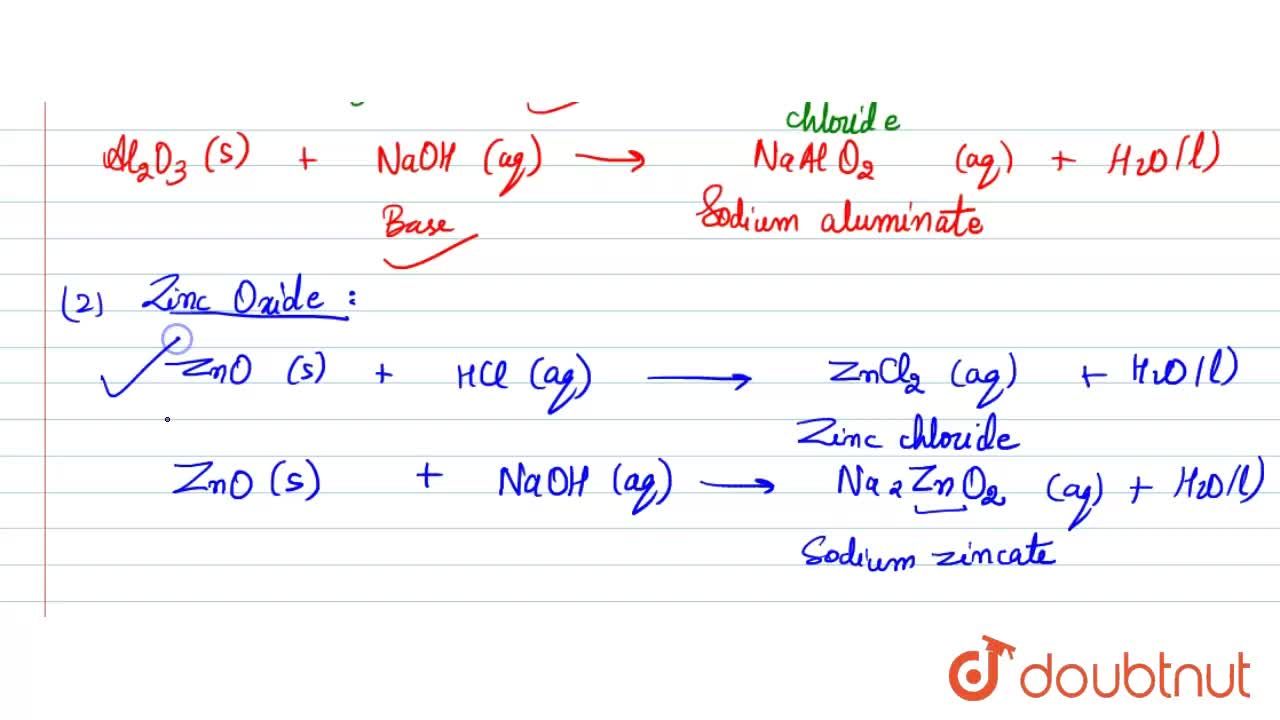
Aluminium oxide and zinc oxide react with both acids are bases to produce salt and water. What are these oxides called ? Write chemical equation in each case.