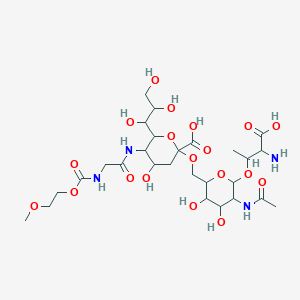
LONQUEX 10 MG/ML SOLUÇÃO INJETÁVEL COM 1 SERINGA PREENCHIDA DE 0,6 ML - LIPEGFILGRASTIM - TEVA (REFRIGERADO) - Ative Medicamentos

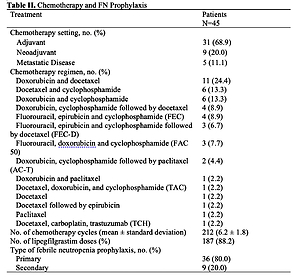
PDF) Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events
A multinational, drug utilization study of lipegfilgrastim use in real-world setting in Europe,Supportive Care in Cancer - X-MOL
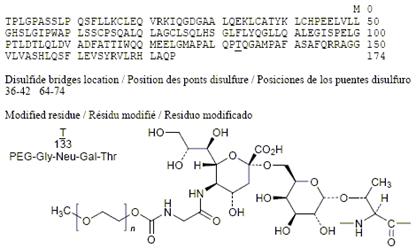
Teva Pharmaceutical has been given a green light by the European Commission (EC) for Lonquex, a rival to Amgen's blockbuster Neulasta. | New Drug Approvals
Teva's Lonquex®(XM22 lipegfilgrastim) Recommended for Approval in the EU for the Reduction of Chemotherapy-Induced Neutropeni