Which of the following statements are correct ? (i) Boron reacts with concentrated HNO3 to form nitric oxide and boric acid (ii) Boron reacts with fused NaOH to form H2O2 and boric

SOLVED: Refer to the neutralization of nitric acid by sodium hydroxide discussed in the Introduction. Calculate the heat of reaction (in kJ) when 40.7 mL of 1.2 M nitric acid reacts with
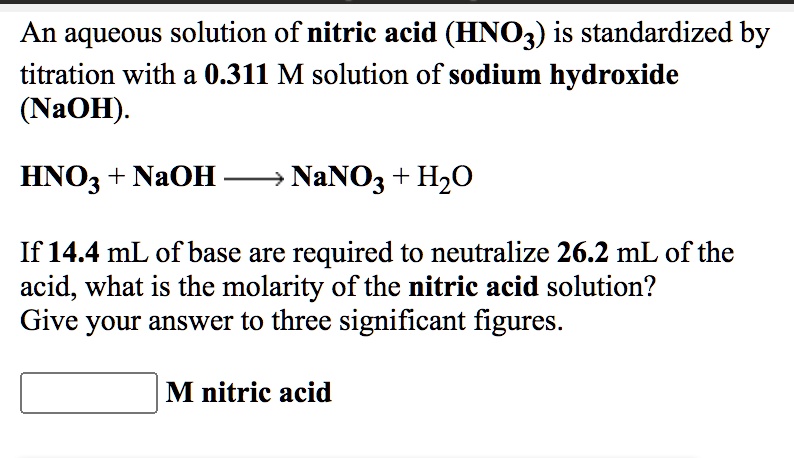
SOLVED: An aqueous solution of nitric acid (HNO3) is standardized by titration with a 0.311 M solution of sodium hydroxide (NaOH): HNOz + NaOH 77 NaNOz + H2O If14.4 mL of base

Ionic equations A chemical equation shows the number of atoms and molecules of the reactants and products. Also shows physical state of reactants and products. - ppt download

The pH titration curves of nitric acid (C 0 a = 0.0002 mol l −1 ) being... | Download Scientific Diagram
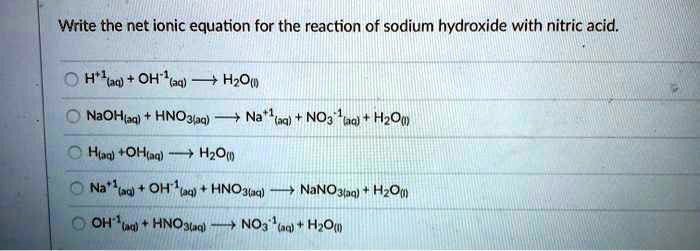
SOLVED: Write the net ionic equation for the reaction of sodium hydroxide with nitric acid: H*laq) OH""(aq) HOu NaOHba) HNO3q) (nq) NO3 HzOw HOm) Hud " +OHiaq) - Itdl) OH'(a4) - HNOglaq)
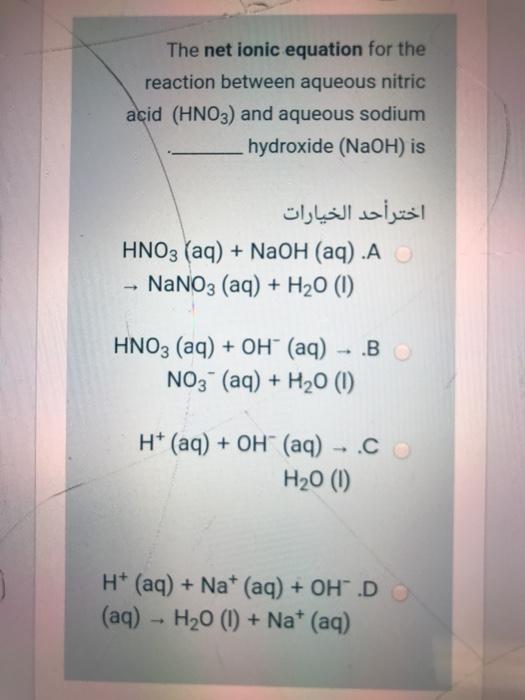
SOLVED: The net ionic equation for the reaction between aqueous nitric acid and aqueous sodium hydroxide is . A) H+ (aq) + HNO3 (aq) + 2OH- (aq) → 2H2O (l) + NO3- (

SOLVED: The net ionic equation for the reaction between aqueous nitric acid (HNO;) and aqueous sodium hydroxide (A) HNO; (aq) OH (aq) NO; (aq) HzO () (B) H* (aq) = OH- (aq) -