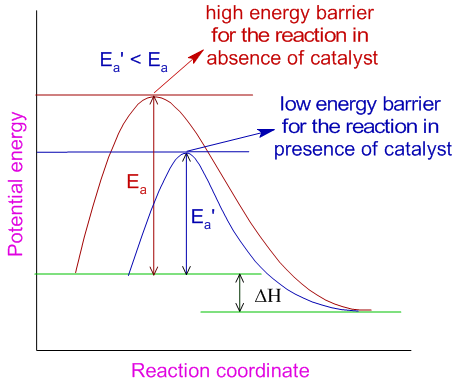
The Rate Law. Objectives: To understand what a rate law is To determine the overall reaction order from a rate law CLE ppt download
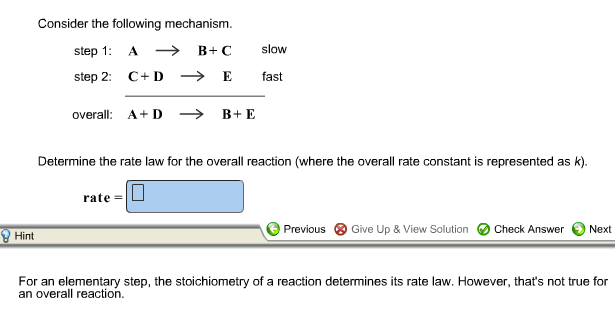
Determine the rate law for the overall reaction (where the overall rate constant is represented as k) - Home Work Help - Learn CBSE Forum
![a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii) a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii)](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SHYtcW5rUVktNDQ=/sd/)
a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii)
![SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order](https://cdn.numerade.com/ask_previews/bea16924-e043-4592-9ba1-7c64b3c6ae29_large.jpg)
SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order

How to Determine the Order of Reaction by Comparing Initial Rates of Reactions | Chemistry | Study.com
![a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii) a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii)](https://i.ytimg.com/vi/Hv-qnkQY-44/maxresdefault.jpg)
a) For a reaction A + B→ P , the rate is given by Rate = k[A][B]^2 (i) How is the rate of reaction affected if the concentration of B is doubled?(ii)