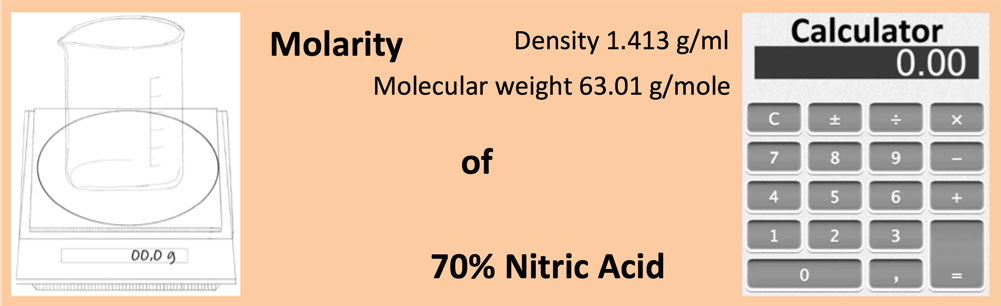
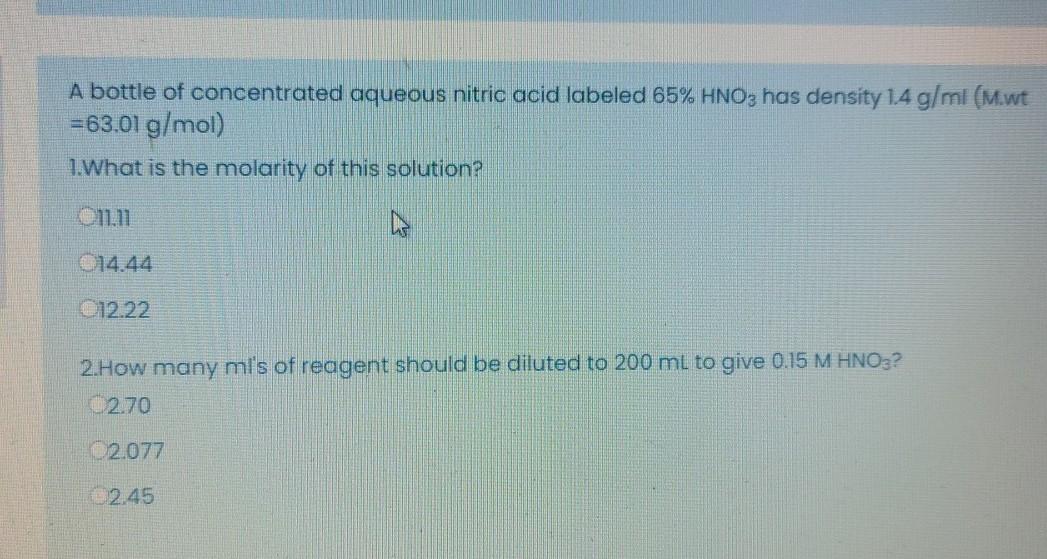
Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

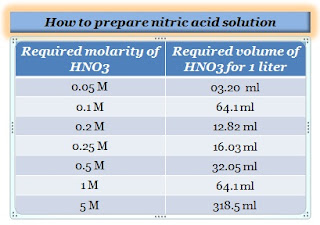
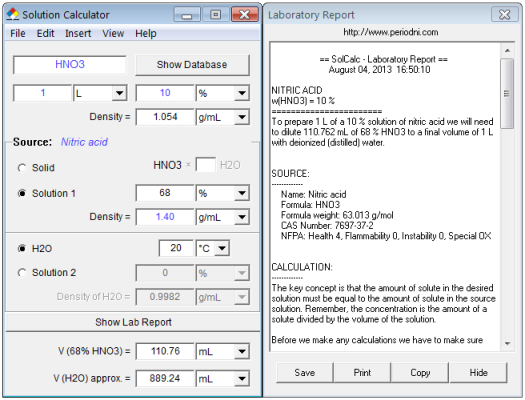
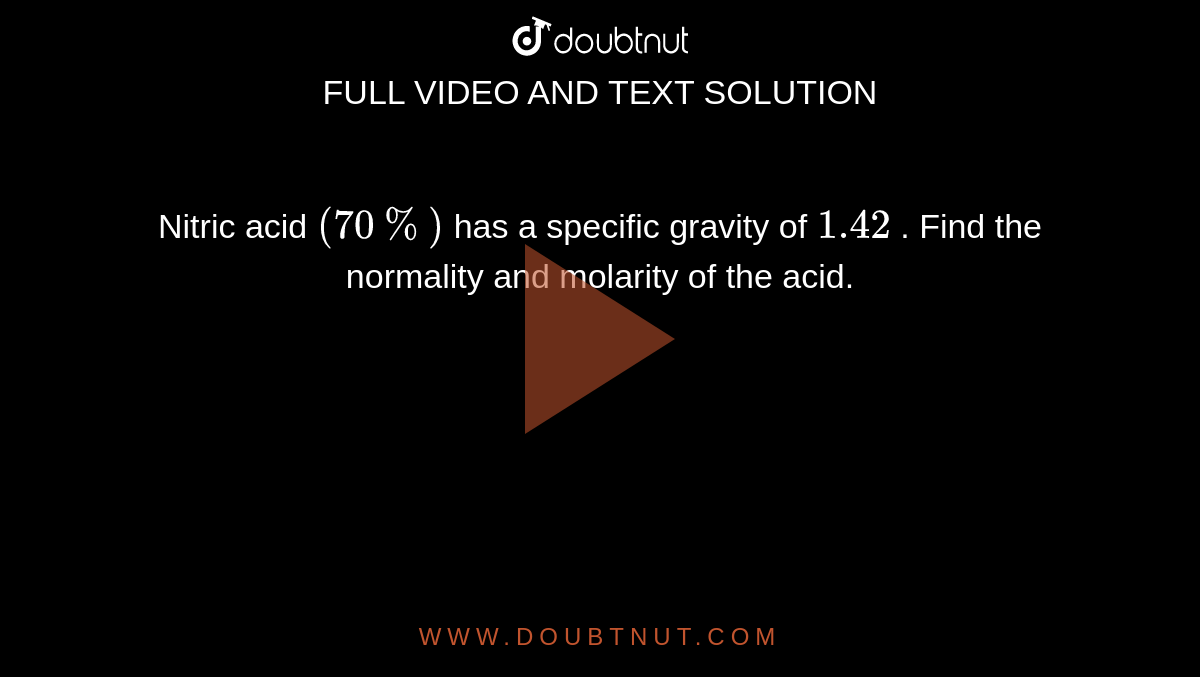
How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3 ? The concentrated acid is 70

Calculate the concentration of nitric acid in moles per litre in a sample which has a density `1... - YouTube
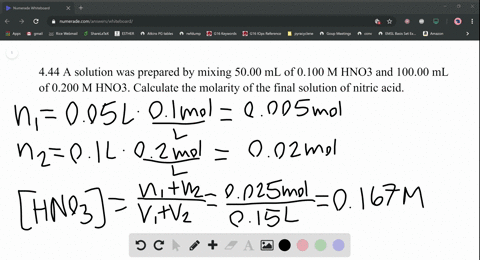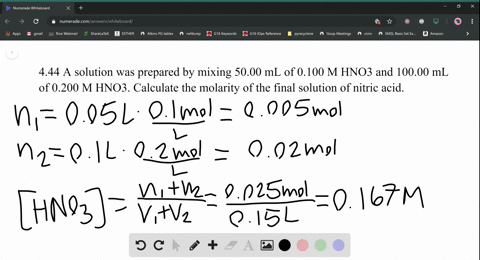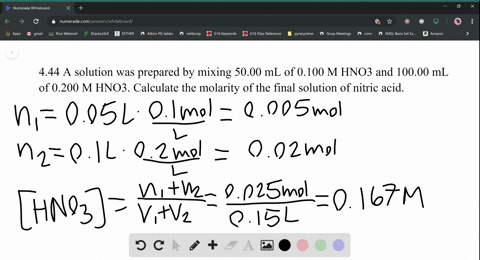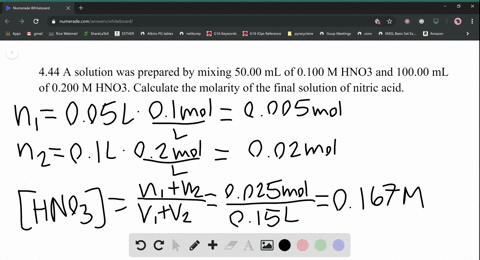
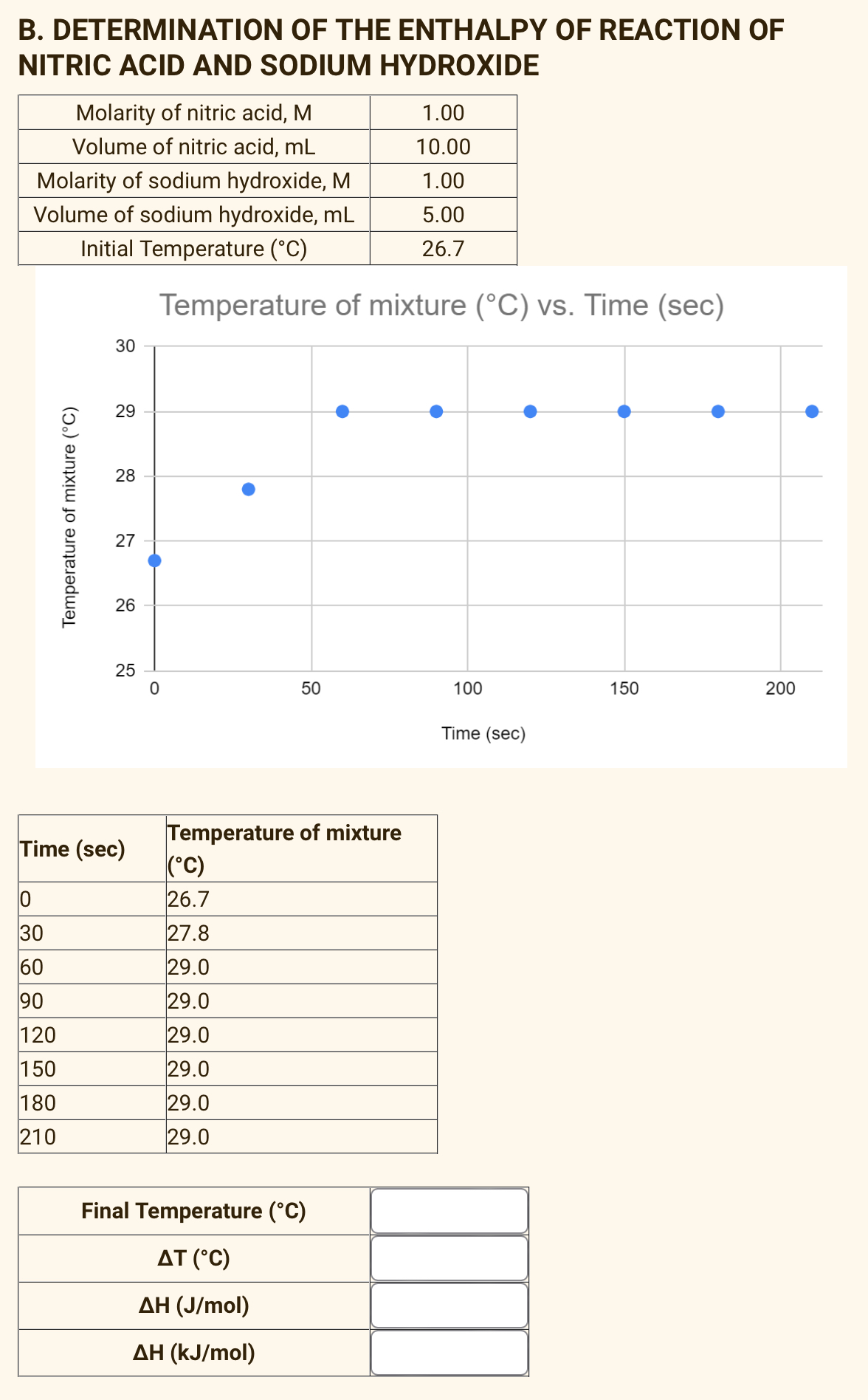
SOLVED:A solution was prepared by mixing 50.00 mL of 0.100 M HNO3 and 100.00 mL of 0.200 M HNO3 . Calculate the molarity of the final solution of nitric acid.

Graph of molarity of nitric acid versus uranium extraction efficiency... | Download Scientific Diagram

How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO(3) solution ? The concentration of nitric acid is 70 % by mass.
Nitric acid, 1 l, glass, CAS No. 7697-37-2 | Reagents for Decalcification | Reagents for Histology | Histology/Microscopy | Life Science | Carl Roth - International