OneClass: Benzoic acid is soluble in diethyl ether but not water, however, benzoic acid is extracted ...

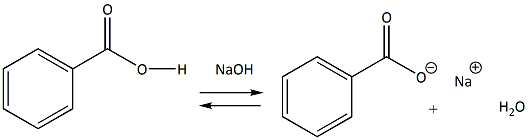
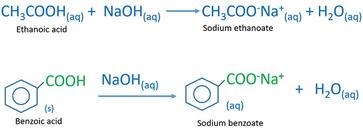
Draw the products of benzoic acid reacting with sodium hydroxide. Draw the products of the pyridine reacting with hydrochloric acid. Use the "+/-" button to add the charge (and H atom).
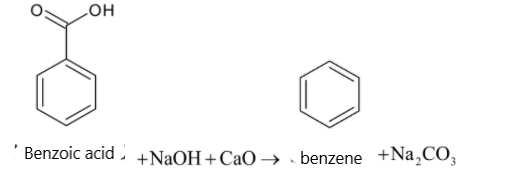
Heating of carboxylic acid with soda lime results in:A. dehydrationB. dehydrogenationC. decarboxylationD. addition of ${{{O}}_2}$
![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://haygot.s3.amazonaws.com/questions/1835171_829774_ans_40c76abc129644cfaff5cbb818338569.png)
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .

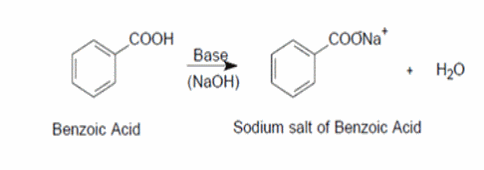
SOLVED: A solution of benzoic acid (HC,HsOz) is titrated using sodium hydroxide: The Ka of benzoic acid is 6.4x 101. Write the balanced equation and the net ionic equation. 2.100.0 mL of

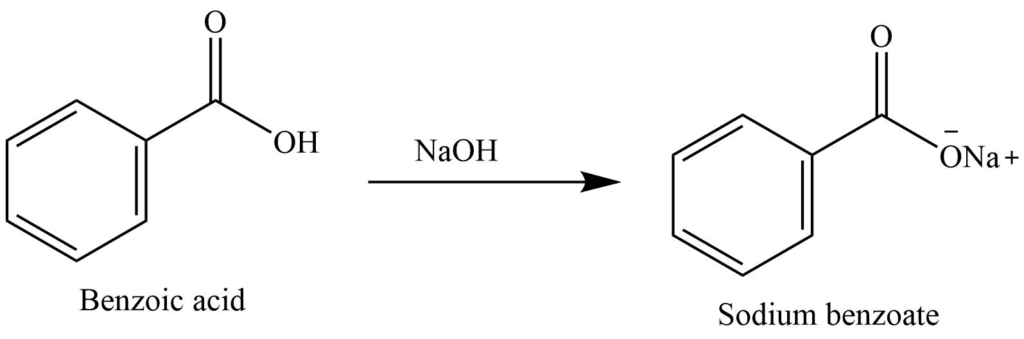
Explain the results for the tube in which 1.0 m naoh was added to benzoic acid. write an equation for this, - Brainly.com

Write a chemical equation that explains the observations on the addition of HCL to the mixture of benzamide and NaOH subsequent to heating. | Homework.Study.com
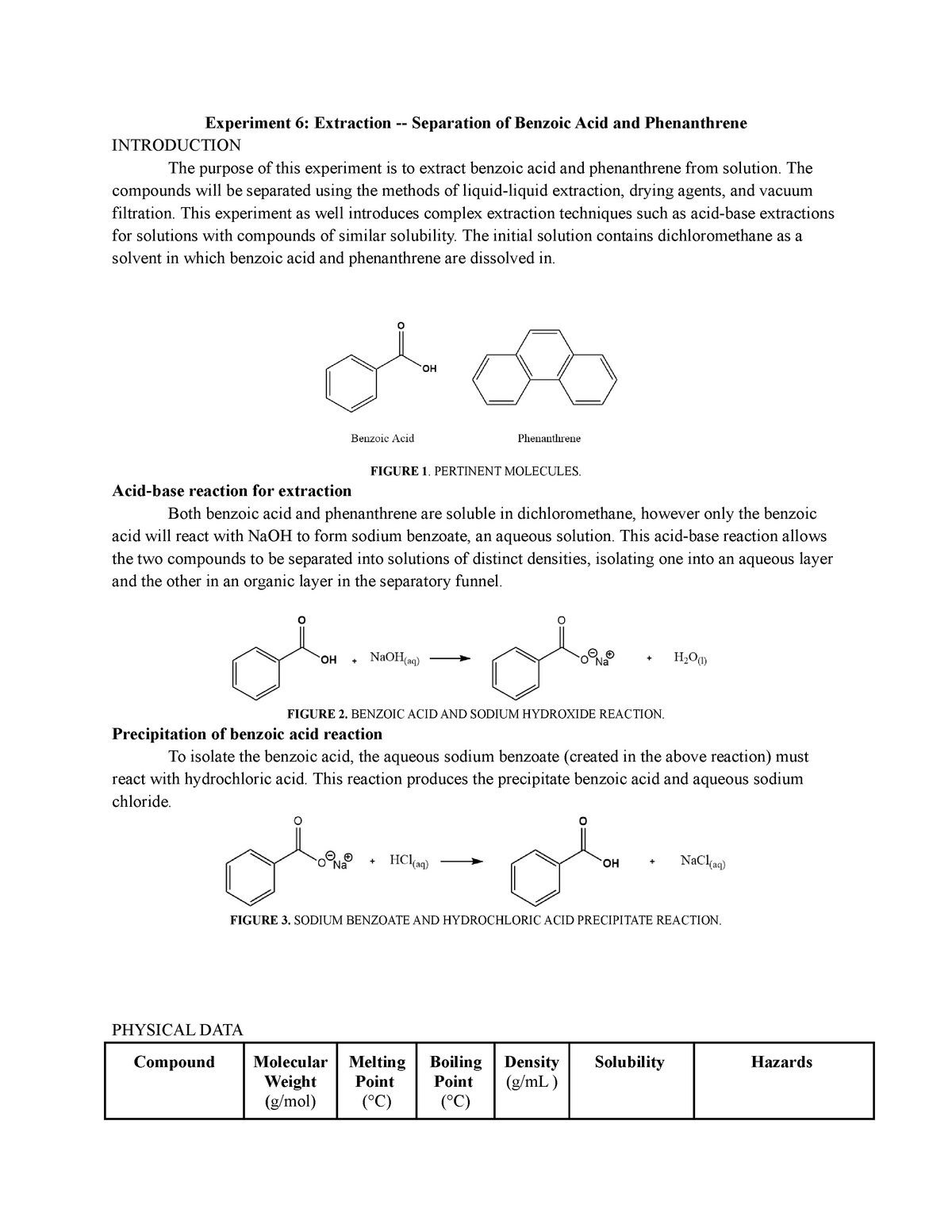
Experiment 6 Extraction - Separation of Benzoic Acid and Phenanthrene - Experiment 6: Extraction - - Studocu

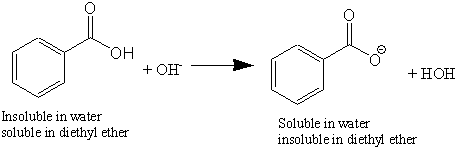
Write a balanced equation for the reaction of benzoic acid with hydroxide ion. Why is it necessary to extract the ether layer with sodium hydroxide? | Homework.Study.com